Behind The Numbers – AtriCure (ATRC)
AtriCure is a medical device company and a leading provider of innovative technologies designed to decrease the global Atrial Fibrillation (Afib) epidemic.
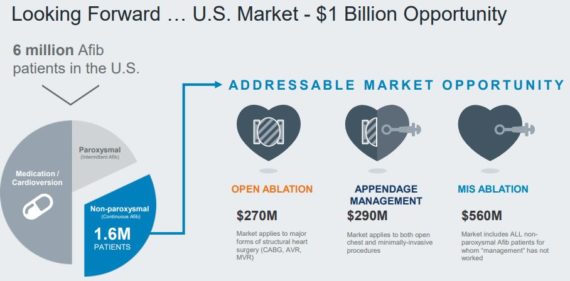
Per the company’s Investor Relations page, approximately 9% of people over 65 suffer from atrial fibrillation, a quivering or irregular heartbeat (arrhythmia) that can lead to blood clots, stroke, heart failure and other heart-related complications. More than 33 million people are affected worldwide, where 2/3 are not addressed by standard of care. Roughly 1.2 million Afib cases are diagnosed annually in the U.S.
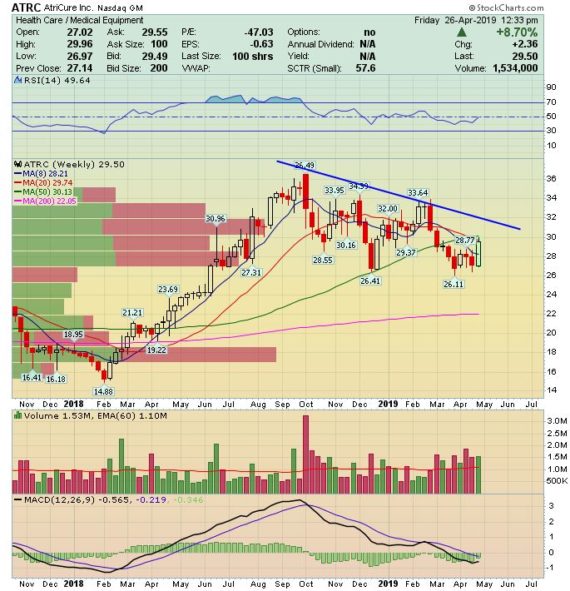
After the close yesterday, the company reported Q1 earnings that beat on both the top and bottom line with management also raising revenue guidance at the low end of the range. Shares are currently higher today by 5%.
-EPS of ($0.20) vs ($0.24) estimate – Beat
-Revenue of $54M vs $52.45M estimate – Beat
-Total Revenue increased 15% (26th straight double-digit quarter)
CEO Michael Carrel would discuss further on the conference call by saying that their appendage management franchise continues to outperform with first quarter growth of 32% Y/Y. They have now sold over 180,000 AtriClip devices worldwide and anticipate this business will be their fastest growing franchise for the foreseeable future.
Piper Jaffray was out with a note saying the most important takeaway from the call, in their view, was the strength in appendage management. Analyst Matt O’Brien adds, “Clearly, surgeons remain highly interested in the Pro-V and Flex-V products launched last year and we think ATRC is going to continue driving upside over the course of this year within this segment. This area is still massively underpenetrated despite the broadening awareness of benefits of LAA management (bolstered by clinical data and society endorsements), so there is a lot of sustainable business still ahead here.”

Management would then discuss their Minimally Invasive (MIS) business, in which they said they experienced some softness in the quarter. They would remind investors that MIS results continue to fluctuate quarterly due to a small number of sites comprising a relatively large portion of the MIS volume. They expect this trend of quarterly volatility to remain until they receive the FDA approval for the EPi-Sense system, and as such, have factored this into guidance for the year.
International
Another bright spot highlighted my management was its international performance, which reported 28% revenue growth in the quarter. CEO Michael Carrel added that in Europe, they saw particular strength from the U.K., France, and Germany with AtriClip and EPi-Sense devices driving the momentum. In Asia, they are now seeing consistent order patterns and expect strength in those markets throughout the year. While international business represents roughly 20% of overall revenue, with investments in the past years, they expect it to be consistently steady and strong grower for the business.
BTIG Research analyst Sean Lavin would comment in a post-earnings note that international sales were solid with strength seen in the UK, France and Germany while Japan continues to be a highly attractive market, particularly for AtriClip. On whether international sales (spurred in part by the rebound in China) might be a disproportionate driver of sales through the year, “We find it encouraging that management appeared bullish on growth being fairly broad-based across segments and geographies.”
Trials
Finally, it should be noted that the company has a handful of ongoing trials in progress.
-They began enrollment in their ICE-AFIB clinical trial in the first quarter of this year. They have 3 sites activated and are on track to add several more throughout this year with additional enrollment following. “The ICE-AFIB trial is a unique opportunity to generate systematic clinical evidence on the safety and effectiveness of concomitant cryo surgery for the treatment of Afib patients undergoing structural heart surgery.”
-They also discussed on the call that they were pleased with the progress in their DEEP AF IDE clinical trial, which received FDA approval to include an additional 40 patients. “As a reminder, DEEP AF provides another alternative for minimally invasive approaches, and we look forward to providing updates as the trial continues to advance throughout 2019.”
-Lastly, regarding its Converge trial (objective is to evaluate the safety and efficacy of the EPi-Sense-AF Guided Coagulation System to treat symptomatic persistent AF patients, refractory or intolerant to at least one Class I and/or III AADs), its last patient was treated in August of last year and all patients are followed for 1 year post-treatment. The company will then work with principal investigators and be in a position to submit the data to the FDA as part of an application for premarket approval of the AtriCure EPi-Sense system.
“As we’ve disclosed previously, for planning purposes, we are anticipating an FDA panel and would expect to disclose the data around the time of that meeting. Until then, we continue to make investments in our training programs. We currently have a team of clinical experts well versed in the EPi-Sense system and who understand EP well. That team will be prepared to ramp up our outreach and education programs quickly upon receipt of the PMA for treatment of persistent and longstanding persistent Afib patient population.”