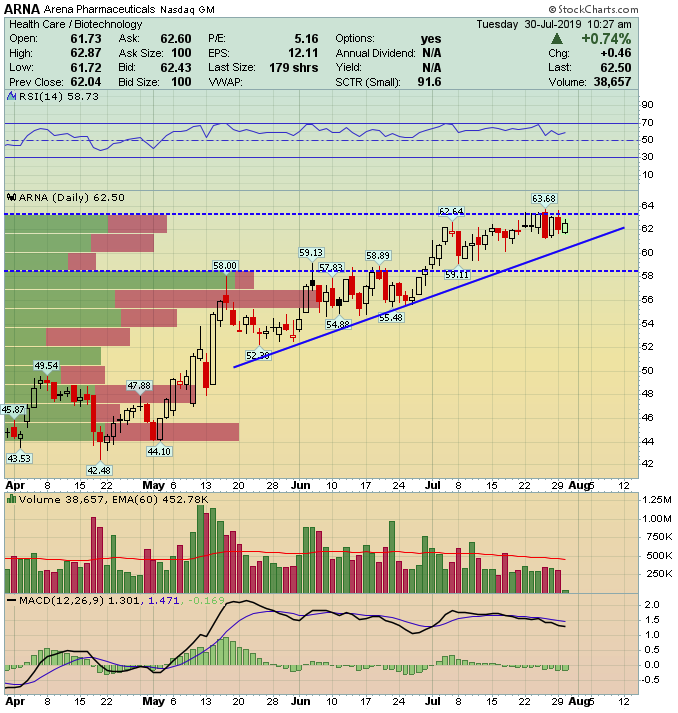
Arena Pharmaceuticals (ARNA) – Promising Novel Therapy Pipeline
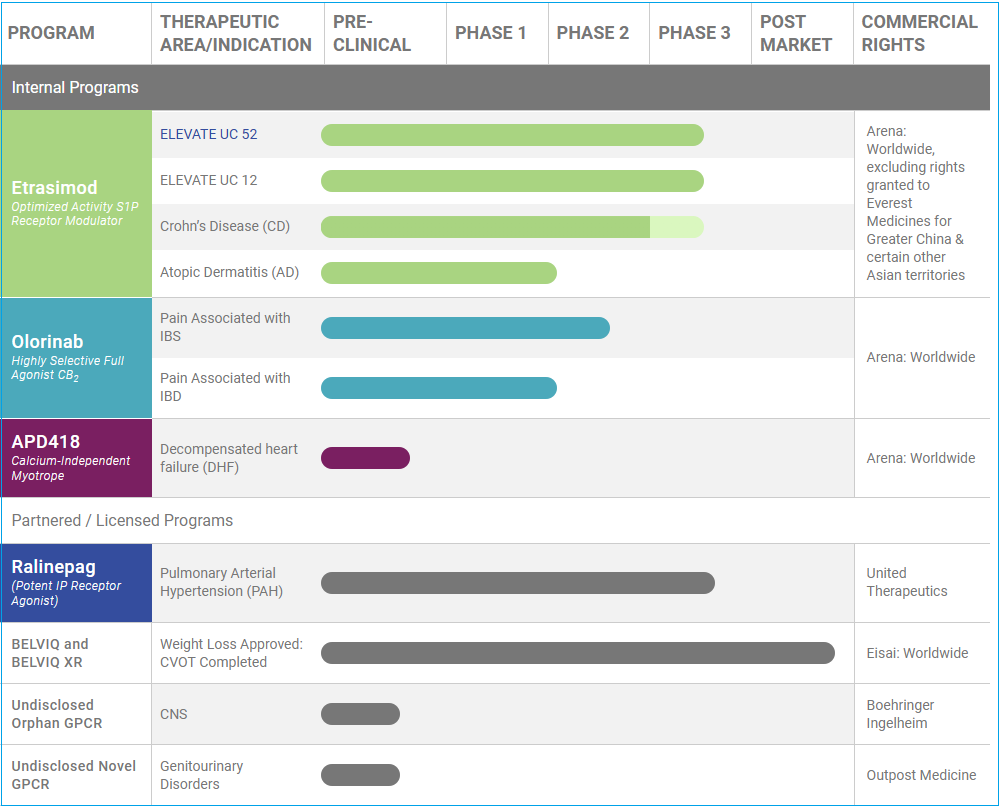
Arena Pharmaceuticals (ARNA) is a San Diego-based biopharmaceutical company focused on developing small molecule drugs across a range of therapeutic areas. Currently, Arena has three primary investigational clinical programs: etrasimod, olorinab and ralinepag. A fourth candidate, ADP-418, is in its pre-clinical evaluation. Lorcaserin, an approved weight management drug is marketed in the United States and South Korea under license to Eisai. The company also has two other yet undisclosed G protein–coupled receptor (GPCR) based therapies in early pre-clinical evaluations with collaboration agreements for development.
Etrasimod
Intended to be a once-daily oral therapy targeting sphingosine-1-phosphate (S1P) receptor modulator to provide systemic and local cell modulation, etrasimod is being tried for immune and inflammatory-mediated diseases such as ulcerative colitis, Crohn’s disease, and atopic dermatitis, the three current indications being developed by Arena.
Ulcerative colitis (UC) is a chronic disease that affects the large intestine in which the innermost lining of the large intestine becomes inflamed leading to ulcer formation on the surface causing a multitude of afflictions. Etrasimod’s selective S1P approach has the potential to improve treatment versus currently available treatment options that have limitations in terms of side effects, patient response, efficacy and administration.
Back in March 2018, Arena completed its Phase II randomized, double-blind, placebo-controlled multinational study returning ‘positive results’ after which two Phase III trials were initiated, ELEVATE UC 52 and ELEVATE UC 12, two concurrent studies that will last 52 weeks with readouts expected to be released simultaneously sometime in 2021. The trial is expected to evaluate the effects of etrasimod, 1mg and 2mg dosage levels, versus placebo on multiple efficacy measures including a 3-component Mayo Score, total Mayo Score (TMS), clinical remission and clinical response in up to 160 patients.
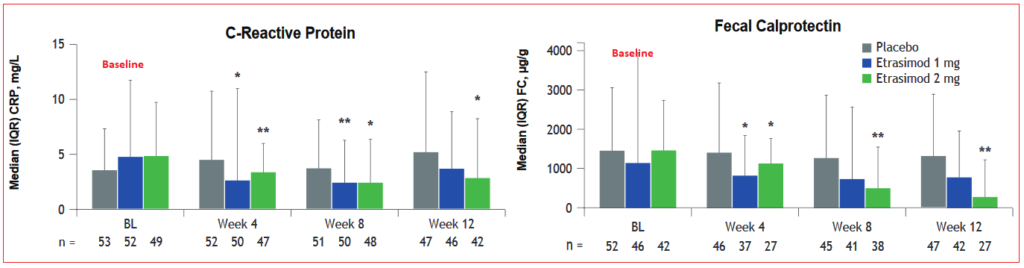
Previously, Phase II study results had shown significantly more patients achieving endoscopic improvement compared to placebo group (43.2% vs 16.3%, respectively), histological improvement (31.7% vs 10.2%), and histological remission (19.5% vs 6.1) at the 12-week point. Mucosal healing was seen in 19.5% of patients treated with etrasimod 2mg compared to just 4.1% of placebo group. Although patients receiving the lower, 1mg etrasimod dose also achieved each endpoint compared with placebo, results did not reach statistical significance.
Crohn’s disease (CD) is a chronic inflammatory condition of the gastrointestinal tract, most commonly affecting the end of the small bowel and the beginning of the colon, but it may affect any part of the gastrointestinal tract. Many patients suffering from Crohn’s disease are prescribed opioids to help with pain management, however this can cause further irritation to the GI tract along with a separate issue, that of addiction to the painkiller.
After encouraging Phase II trials, Arena is planning Phase IIb/III trials which should provide results a few quarters after ELEVAT-UC colitis studies, by design.
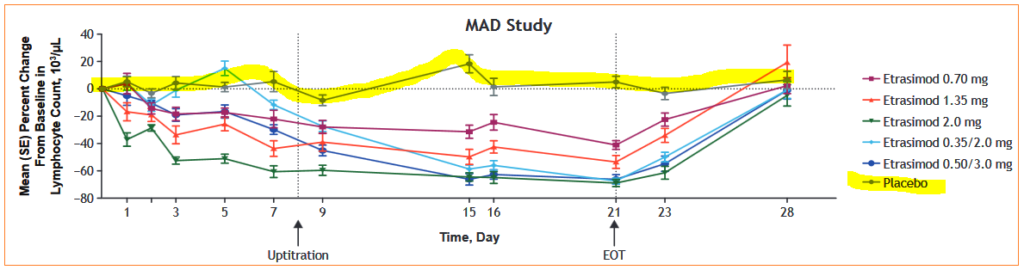
In its Phase I trials, a dose-dependent reduction in total peripheral lymphocyte count was observed with estrasimod versus placebo. When administration was discontinued, lymphocyte levels either increased back towards the baseline, or returned to original levels. Doses of less than 1.0mg had “little or no effect” on T-cell counts however higher dose levels of 3.0mg and 5.0mg reduced total T-cell counts when compared to placebo group. Overall, the drug was well-tolerated in healthy test subjects.
Atopic dermatitis (AD), or eczema, is a condition with varying symptoms, most often manifesting as dry skin, severe itching, patches, swollen skin and raised bumps which may leak fluid. It’s common in children but can occur at any age and is typically chronic, tending to flare periodically. Currently, there isn’t a cure for AD but treatments and self-care measures can relieve itching and help minimize new outbreaks.
Management is planning to initiate a Phase II study in AD by the end of 2019 with data sometime in 2020. During Phase II OASIS trials for ulcerative colitis, patients with eczema manifestations had responded to therapy as the S1P receptor subtypes also modulate other biological responses.
Olorinab
An internally discovered investigational drug candidate, olorinab is being explored for development in several indications as a peripherally acting (primary mechanism of action outside of the central nervous system), highly-selective, full agonist of the cannabinoid type 2 receptor (CB2): compared to CB1, CB2 does not cause psychoactive adverse effects. The treatment’s initial focus is on nervous system pain associated with gastrointestinal disorders.
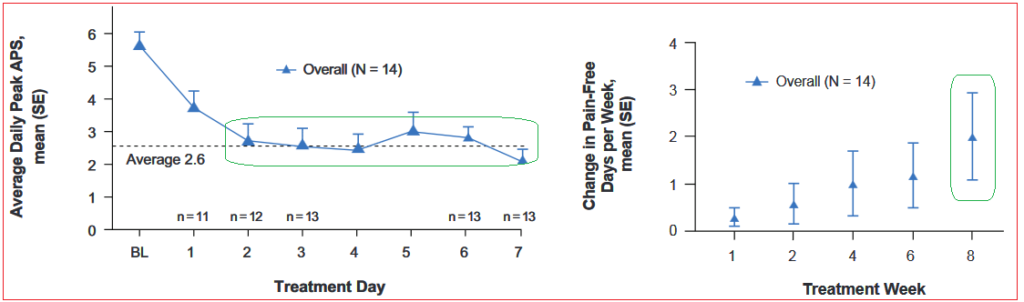
Olorinab is targeting pain management for two indications, irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD). Early results have shown clinical response in Average Abdominal Pain Scale (AAPS) with a ≥30% reduction in 85% (11/13) of all subjects with evaluable data at Week 4, and 100% (11/11) at Week 8. At Baseline, no subject had a pain-free day, this increased to a mean of two pain-free days per week in Week 8.
On July 25th, Arena announced the first subject doses in CAPTIVATE Phase IIb trial for IBS, evaluating three dose levels over a twelve-week period in approximately 240 patients with various IBS-related symptoms. The primary endpoint is improvement in the weekly AAPS from baseline.
ADP418
Revealed in late 2018, APD418 is intended to be a first-in-class option for decompensated heart failure (DHF). The selective βeta3 adrenergic receptor (AdrR) antagonist is designed to improve cardiac contractility (the ability of the heart muscle to contract) with minimal effect on heart rate and blood pressure. While enhancing performance, this treatment tends to avoid adverse events associated with current therapies.
Decompensated heart failure is characterized by an increase in symptoms such as difficulty breathing, intolerance to exertion, swelling of the limbs, and fatigue. The mortality rate remains high as DHF is a complex disease; it is also the leading cause of hospitalization for those over 65 years of age.
Early testing on non-human subjects has shown improvement ventricular mechanical efficiency, supporting development of ADP418 for in-hospital treatments of patients with chronic heart failure.
Arena plans to file an Investigational New Drug (IND) application with the FDA in 2H2019.
Ralinepag
Potentially a ‘Best-in-Class’ therapy for pulmonary arterial hypertension (PAH), ralinepag is differentiated from current treatments in that it is a next-generation, oral medication compared to current inhalation therapies. Trials have progressed to Phase III stage in which its impact on morbidity, mortality and exercise capacity is being evaluated.
On January 24th, Arena announced closing of a license agreement with United Therapeutics (UTHR) that was entered into in late November 2018. The deal grants United Health an exclusive global license for ralinepag and, in return, Arena received an $800M upfront compensation with up to $400M in future milestone payments as well as tiered, low double-digit royalties on net sales.
In its primary Phase II trials, ralinepag had shown a statistically significant absolute change from baseline in pulmonary vascular resistance (PVR) compared to placebo. Patients showed a 29.8% improvement in PVR compared to placebo arm and a 20.1% improvement from the baseline. Subsequently, the open-label extension study demonstrated sustained improvements in the original patients that were switched from placebo to ralinepag, with results achieving a similar magnitude of improvement. Moving forward trials will be conducted and reported by United Therapeutics.
As of March 31st, Arena had $1.1B in cash, including United Therapeutics’ payment, with over $400M in milestone payments expected in the future. In FY2018, R&D plus G&A expenses were around $163M which their current bankroll can sustain for quite a long time. While the rest of 2019 will be muted in terms of data releases, 2020 will likely see at least one readout, and possibly an update on ralinepag (depending on United Therapeutics’ timeline). Internally, management has stated they are continuing to scale the enterprise with buildout of their commercial and medical affairs teams. Management believes they are uniquely positioned to deliver on the promise of their products with best-in-class products and a potentially high-value pipeline that has limited downside risk.