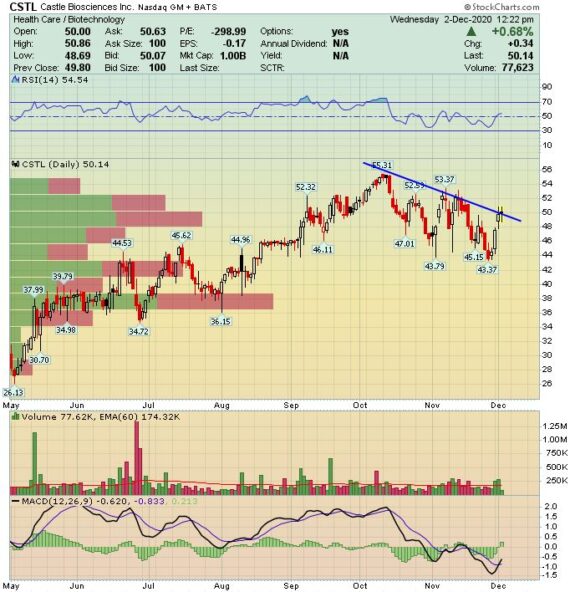
Castle Biosciences (CSTL) – Decisions Decisions
Castle Biosciences (CSTL) is a market leader in molecular diagnostic testing for multiple types of skin cancer. The company’s tests provide clinically actionable, tumor-specific genomic information to enable more accurate treatment plan decisions. KeyBanc analyst Paul Knight highlighted in his initiation note on November 9th that growing revenue at an expected rate of 34-40% the next three years, the company operates at one of the highest industry gross margins at 85.9%.
The company’s “DecisionDx” tests are outlined below:
DecisionDx Melanoma – A test for invasive cutaneous melanoma. DecisionDx®-Melanoma, is a proprietary gene expression profile, or GEP, test that more accurately predicts a patient’s risk of metastasis or recurrence as well as sentinel lymph node positivity.
DecisionDx SCC – This is their GEP test that predicts the risk of metastasis for patients with cutaneous squamous cell carcinoma who have one or more risk factors.
DecisionDx UM – A test that predicts the risk of metastasis for patients with uveal melanoma, a rare eye cancer.
DecisionDx DiffDx – The company’s latest proprietary gene expression profile test. It is designed to provide a highly accurate, objective result to aid dermatopathologists and dermatologists in characterizing suspicious pigmented lesions.

Q3 Earnings Recap
-EPS of ($0.23) vs ($0.11) estimate – Miss
-Revenue of $15.2M vs $14.99M estimate – Beat
-Revenues increased 3% Y/Y
-7% Delivery Increase in DecisionDx Melanoma Tests
On the conference call, CEO Derek Maetzold highlighted that the company provided 4,404 DecisionDx-Melanoma test reports, a 46% increase Q/Q. He remarked that they delivered this increase even as their analysis of third-party data told them that in the third quarter cutaneous melanoma diagnoses decreased by approximately 12% Y/Y. Additionally, they also captured additional clinicians to order their Melanoma test for the very first time. Now, they did say they expect COVID to continue to impact DecisionDX melanoma volume in Q4 and into 2021 due to delays in the overall number of patients diagnosed with cutaneous melanoma.
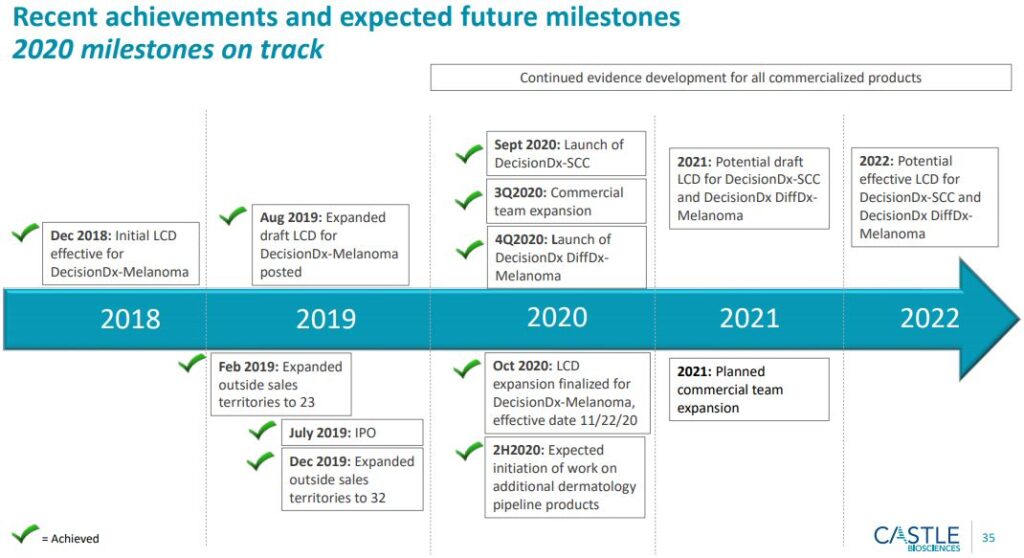
Turning to reimbursement, the company accomplished an important DecisionDx-Melanoma milestone in the quarter. “The Medicare Administrative Contractor, or MAC, Palmetto MolDX issued a draft, local coverage determination policy or LCD in August 2019. The final positive expanded LCD and the accompanying billing and coding article are approached in October 2020. The effective date for the Palmetto LCD and the billing and coding article is November 22 2020. Importantly, this LCD article was nearly identical in terms of coverage to the draft August 2019 LCD. Meaning that the expanded LCD provides reimbursement for the significant majority of Medicare beneficiaries, whose clinicians order DecisionDx-Melanoma as part of their patient management plan.”
Separately, the company successfully executed on a launch of DecisionDX-SCC, which became commercially available on August 31st. The company has submitted their technical assessment dossier for Medicare coverage to Palmetto MolDX and in the third quarter, Palmetto confirmed that it was accepted as complete. Accordingly, the company expects a draft local coverage determination policy to be posted in 2021 with finalization expected approximately 12 months following the draft. “As a reminder, we expect Medicare to be our largest payer for DecisionDX-SCC, as a typical age and time of diagnosis of SCC is near 17 years.”
Canaccord Meetings
On November 30th, Canaccord Genuity analyst Max Masucci was out with a Diagnostics note following meeting with CEO Derek Maetzold, CFO Frank Stokes, and Executive Director of IR Camilla Zuckero. The analyst commented that for those looking to fortify an existing position or for investors that have been awaiting an attractive entry point, he believes now is an opportune time to strike. Additionally, here are some of his specific takeaways:
-CSTL’s flagship DecisionDx-Melanoma test has achieved ~14% penetration in the ~5 years since launch, but until December 2018, CSTL was operating with just 14 direct sales reps. After two controlled sales force expansions (CSTL’s current sales force includes 32 reps focused on DecisionDx-Melanoma, DecisionDx-UM, and DecisionDx-SCC, and ~10 reps exclusively focused on Diff-Dx), CSTL should see an acceleration in its penetration rate. With this in mind, CSTL believes that a ~65-85% penetration rate for DecisionDxMelanoma should be achievable within the next 10 years, and may prove to be a conservative estimate.
-CSTL’s DecisionDx-UM test has achieved 85%+ penetration since its 2019 launch and is included in NCCN guidelines. CSTL sees an opportunity to drive similar penetration levels for its three other commercially launched tests, where CSTL is just ~3% penetrated.
-The average age of a patient diagnosed with SCC is ~71 and Medicare patients represent ~65-70% of CSTL’s addressable population. CSTL has submitted its SCC data to CMS and the MACs for consideration, and the submission has been labeled as “complete.” CSTL (and we) continue to expect draft Medicare coverage to be posted in late 2021, but this may be conservative thinking. Roughly 8-15 months after the draft Medicare coverage is posted, DecisionDx-SCC should be granted final coverage.
-CSTL has launched its DiffDx test through its specialized 10-rep “SWAT Team” sales force, which will target sales to dermatopathologists. CSTL expects its DiffDx sales force to compete favorably with Myriad Genetics’ dermatology sales force, which commercializes the myPath Melanoma test, but is currently evaluating strategic alternatives. Given the adjacent nature of the dermatopathologist call point, CSTL expect DiffDx’s market penetration to lag the pace observed for SCC. That said, CSTL already holds relationships with 1,800+ dermatopathologists. Early data suggests that DiffDx’s performance is super to myPath Melanoma. DiffDx’s ability to reduce the “indeterminate range” should serve as key selling points for CSTL’s sales reps.