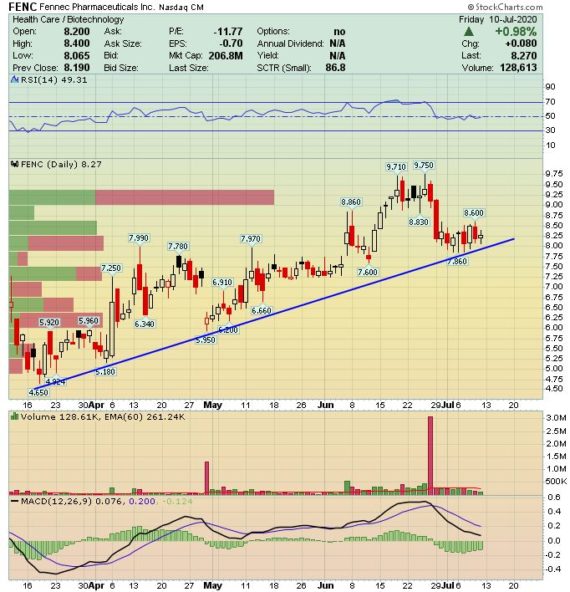
Catalyst Watch – Fennec Pharmaceuticals (FENC)
Fennec Pharmaceuticals (FENC) is a biotech company focused on the development of PEDMARK for the prevention of ototoxicity from cisplatin in pediatric patients.
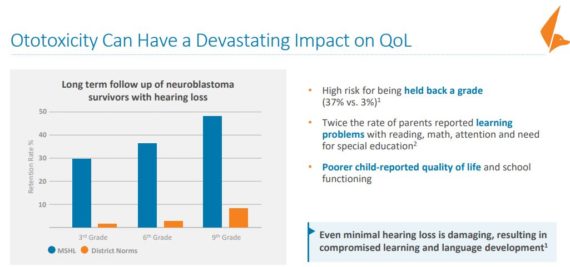
Cisplatin and other platinum compounds are essential chemotherapeutic agents for many pediatric malignancies. Unfortunately, platinum-based therapies cause ototoxicity, or hearing loss, which is permanent, irreversible and particularly harmful to the survivors of pediatric cancer. In the U.S. and Europe, it is estimated that, annually, over 10,000 children may receive platinum-based chemotherapy. The incidence of ototoxicity depends upon the dose and duration of chemotherapy, and many of these children require lifelong hearing aids. There is currently no established preventive agent for this hearing loss and only expensive, technically difficult and sub-optimal cochlear (inner ear) implants have been shown to provide some benefit.
Back on April 13th, the company announced that the FDA has accepted for filing and granted Priority Review for the company’s New Drug Application (NDA) for PEDMARK™. The FDA set a Prescription Drug User Fee Act (PDUFA) action date of August 10th.
Prior Studies
The company has evaluated PEDMARK in multiple clinical trials, including two positive Phase 3 studies, which showed efficacy for reducing CIHL in patients < 18 years of age. These studies were The Clinical Oncology Group (COG) Protocol ACCL0431 and SIOPEL 6.
Per Cantor Fitzgerald, in the ACCL0431 Phase 3 study, patients receiving STS after cisplatin reduced risk of hearing loss (48%) vs the cisplatin only arm. “What we found striking was that patients <5 years of age had a 70% reduction in risk of hearing loss, the group we believe is at highest risk for experiencing neurocognitive and psychosocial delays as these patients are in the early stages of speech development.”
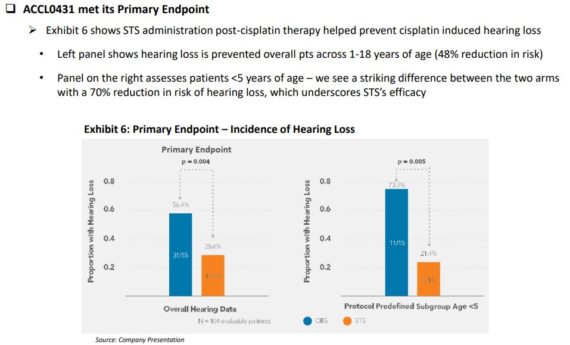
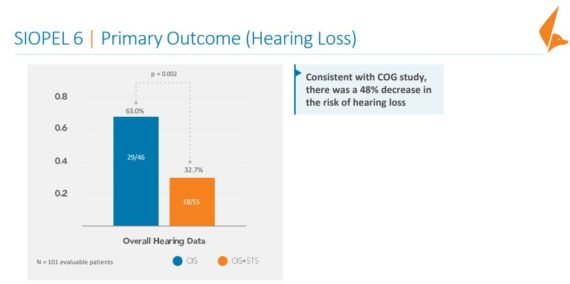
Cantor Fitzgerald added that although clear efficacy was observed in this study, patients were not stratified by tumor risk and localized/disseminated disease, which confounded outcomes for the secondary endpoints of Overall Survival (OS) and Event Free Survival (EFS). However, a post hoc analysis showed the control arm OS curve with disseminated disease did not coincide with historical data and was similar to localized disease, whereas the STS plus cisplatin arm did, suggesting a higher risk cohort. That said, the subsequent Phase 3 SIOPEL 6 study risk-stratified a more homogeneous sample, which in addition to showing a 48% risk reduction for hearing loss in the STS plus cisplatin arm (similar to ACCL0431 P3 results), no difference in EFS/OS were observed and therefore STS did not compromise cisplatin efficacy while it did achieve the desired CIHL therapeutic benefit.
Analyst Commentary
Wedbush – Analyst David Nierengarten said that given the strength of the clinical data shown to date, rarity and high unmet need of the pediatric disease, and favorable regulatory designations awarded, “We view downside risk as limited.” As such, they would be buyers of PEDMARK shares ahead of eventual approval, and see FENC as an attractive M&A candidate post-approval, particularly if a broad label is granted.
H.C. Wainwright – Analyst Raghuram Selvaraju anticipates timely approval for PEDMARK in the U.S. and subsequent launch into a highly specialized market, with the sales effort focusing on pediatric oncologists. From their vantage point, Fennec’s solid cash position and the niche nature of the target market for PEDMARK should enable the company to execute an effective launch, notwithstanding the restrictions currently in place on face-to-face sales activities due to the social distancing requirements necessitated by the COVID-19 pandemic.
In addition, European approval should follow shortly after the U.S. decision. In August 2018, Fennec’s pediatric investigation plan received a positive opinion from the European Medicines Agency (EMA), which establishes a path forward for PEDMARK in Europe with up to 10 years of data protection. Fennec’s MAA for sodium thiosulfate, which was submitted in February 2020, is slated to be reviewed via the EMA’s Paediatric-use marketing authorization (PUMA) pathway. “We expect European approval within a few months of the August 2020 U.S. approval decision—possibly by the end of 2020.”
Catalyst Calendar