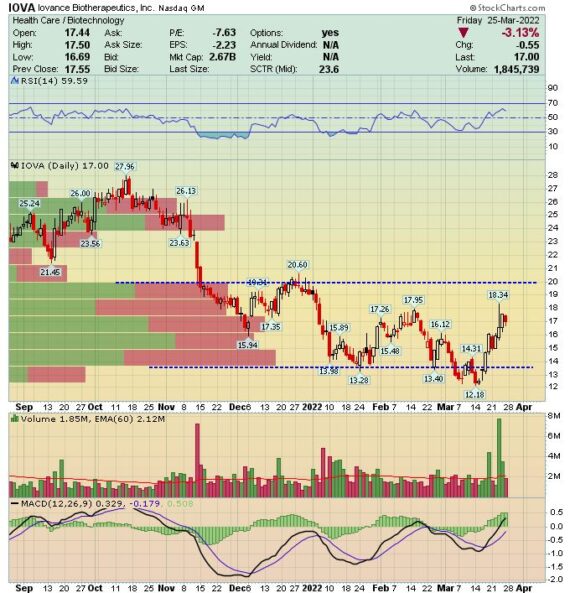
Catalyst Watch – Iovance Biotherapeutics (IOVA)
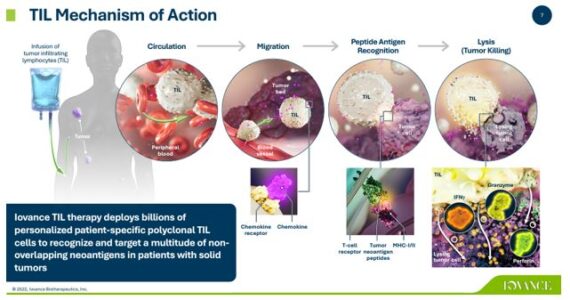
Iovance Biotherapeutics (IOVA) is a clinical-stage biopharmaceutical company that is the front-runner in a branch of cell therapy, known as Tumor Infiltrating Lymphocytes (TIL), for the treatment of patients with cancer. TIL technology is where activated immune cells are extracted out of a tumor biopsy, expanded ex-vivo, and then readministered to the patient. The company’s lead product candidate is called lifileucel, a TIL-based product for which the company has presented data for both metastatic melanoma and for pre-treated metastatic or persistent cervical carcinoma.
Last Friday, we received a private message/comment from a client asking what potential catalysts are on the horizon for this company. Simply put, the key outstanding item for IOVA is the ability to file a Biologics License Application, or BLA, for lifileucel for melanoma and the company has stated this will occur sometime in the 1H of this year. This certainly aligns with the option flow we have been seeing in April, May, and June strikes. Clients can check out JaguarScan for a full breakdown.
Per Interim CEO Fred Vogt on the Q4 conference call: “For our lead TIL therapy, lifileucel, our top priority remains our planned BLA submission in metastatic melanoma. We have continued ongoing work developing and validating our potency assays. We have also engaged discussions with the FDA during the second half of 2021, and we are confident in our current guidance for the anticipated BLA submission during the first half of 2022.”
An interesting development started back on January 10th when Iovance announced that Raj K. Puri, M.D., Ph.D., would be joining the company as Executive Vice President, Regulatory Strategy and Translational Medicine. In the press release, it indicated that Dr. Puri would begin his employment with the company toward the end of the first quarter. For more than 19 years, Dr. Puri served as the Director of the Division of Cellular and Gene Therapies in the Office of Tissues and Advanced Therapies at the FDA in its Center for Biologics Evaluation and Research.
On January 25th, Stifel was out with a note revisiting their Iovance thesis following this news. Analyst Benjamin Burnett said he thinks this is a significant event that has positive implications for the long-term commercial prospects of lifileucel. “We’re surprised that the stock hasn’t traded more strongly around this announcement; we’re of the view that this tilts what we had previously viewed as a 50/50 situation more positively, and therefore see this as a buying opportunity. Our view is also aided by KOL discussions with a prior FDA member (who previously worked within Dr. Puri’s division at the FDA), who didn’t offer specific opinions around Iovance’s situation, but did hold Dr. Puri in high regard. When Dr. Maria Fardis left**, seemingly abruptly, we felt that leadership changes signaled something negative about this situation. Making a high-quality hire in Dr. Puri helps assuage some of our concerns here. We’re now of the view that this situation likely gains resolution and ascribe a 60% probability-of-success (up from 50% prior) to lifileucel in our model. That, along with the broader sector pullback, underscores our upgrade; our new target price is $25/share.”
**Maria Fardis announced her resignation on May 19th, 2021 to pursue other opportunities. No permanent CEO has been announced yet.
While we await an FDA update. H.C. Wainwright analyst Joseph Pantginis highlighted that Iovance has made substantial efforts towards establishing manufacturing infrastructure. This includes construction of its iCTC new facility, the addition of clean rooms, and acquiring all obligatory product processing equipment. Moreover, the company is pursuing numerous initiatives to improve product marketing, accelerate site opening and training, and increase patient outreach and awareness. Much of this preparation continues in parallel to acquiring FDA approval for a TIL potency assay.
Then, beyond the BLA submission, management said it expects to provide data from its pivotal melanoma cohort 4 study in connection with the BLA submission. Regarding the front-line melanoma program, speculation is that we could see additional data either mid-year (possibly at ASCO) or in the 2H22. Separately, regarding the lifileucel cervical cancer program, management is engaged in discussions with the FDA, and analysts estimate that an update on the regulatory path could come when/if the potency assay is resolved. Other updates that could come in 2022 are head-and-neck data and an eventual announcement of a new CEO. Mizuho was out last week with a Hematology and Oncology Catalyst Tracker note where a timeline for Iovance was presented:
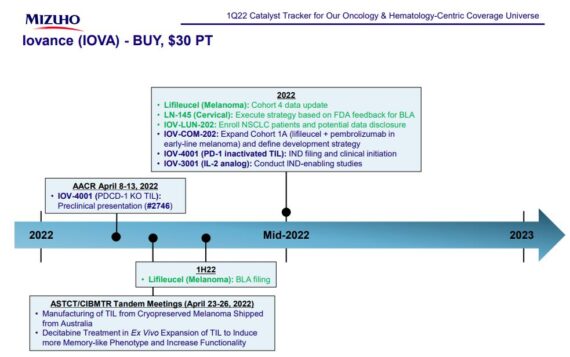
Finally, the next notable conference taking place is the American Association for Cancer Research (AACR), which will be held from April 8th – 13th. As it relates to IOVA, they will have in-person poster presentations on April 11th and 12th.