Global Blood Therapeutics (GBT) – 2Q Earnings Preview
Ahead of Global Blood Therapeutics’ (GBT) second quarter earnings release on August 5th; two recent analyst updates may be indicating a path higher for Oxbryta sales. Launched shortly after receiving FDA approval in November 2019, Oxbryta is a treatment for sickle cell disease (SCD) for patients aged 12 and older. Our original coverage in this name goes back to November 2017 where a more detailed presentation was made.
1Q2020 Recap
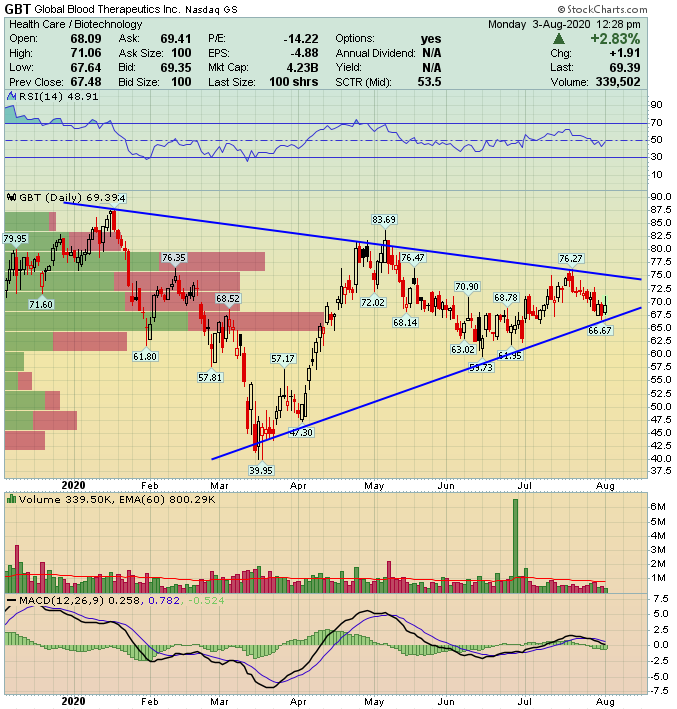
In 1Q2020 management noted that new script additions had declined by as much as 60% from March highs. This decline is a direct result of COVID19 related lockdowns that have affected all healthcare sectors, regardless of how crucial they are to the well-being of patients suffering from hundreds of other conditions and illnesses. First quarter sales were reported at $14.12M at which time management also stated the script decline noted above. Prescriptions reached 1,650 new patients in 1Q2020, totalling 2,000 since its launch.
Analyst Previews
In early July Stifel noted that based on a survey done in June, in-person visits to healthcare professionals were 60% of normal levels, compared to around 40% of normal amount of visits during the months of April and May. The analyst estimates that this increase could result in 2Q2020 Oxbryta sales of $23.5M, above Street expectations of $17.4M.
Aside from patient visit increases, there is new interest and effort in initiating patients on Oxbryta via virtual consultations which, along with geographical rollback or easing of “shelter in place” severity, support the analyst’s view of higher upcoming total scripts.
Separately, Piper Sandler conducted a key opinion leader (KOL) survey at the end of July with resulting data that also points to growing Oxbryta usage during the quarter. Multiple consultations with physicians in the field captured 194 sickle cell disease patients treated with Oxbryta, compared to 144 in 1Q. This implies Oxbryta market penetration having increased from 3.9% to 5.2% sequentially, with improving reimbursements from payers as well. The analyst raised his 2Q2020 sales estimates from $17.5M to $23.3M, and FY2020 estimate from $82.8M to $100M versus Street view of $88.8M.
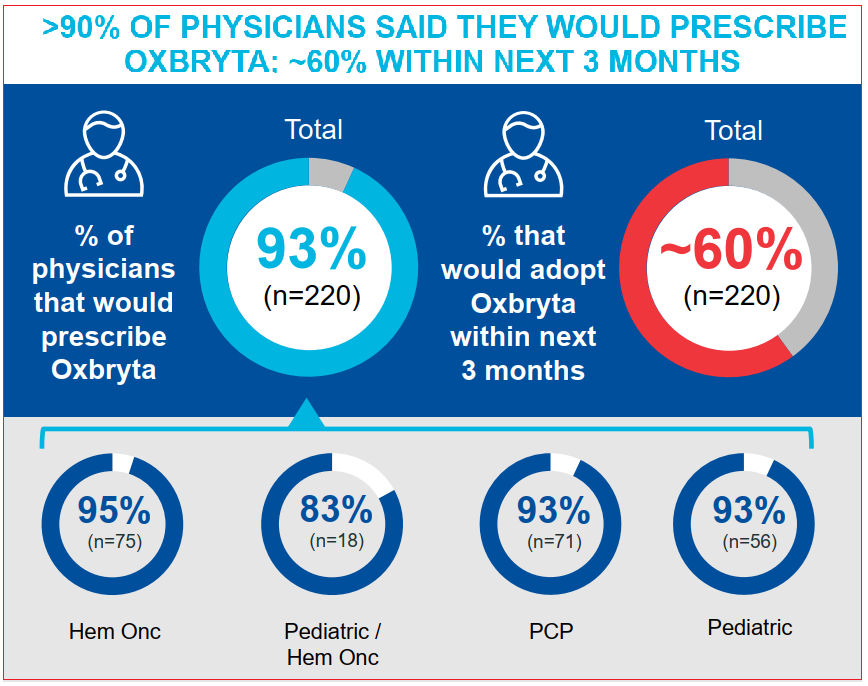
And from the company’s own presentation done in late-June, it is clear that Oxbryta interest remains high among doctors who have patients with sickle cell disease. Over 90% of surveyed physicians indicated they would prescribe the therapy; nearly two thirds would do it within the next three months.
Although Global Blood Therapeutics is conducting trials for Oxbryta label expansion to treat children aged 4 to 11 years, updates are not expected until late 2020. At this time their upcoming earnings release remains the near-term catalyst.