Global Blood Therapeutics (GBT) – Oxbryta Clarity
If we first go back to November 2019, we will find that the FDA granted accelerated approval to Global Blood Therapeutics (GBT) and Oxbryta for adults and pediatric patients 12 years of age and older with sickle cell disease. According to fda.gov,
“Efficacy was evaluated in 274 patients with sickle cell disease in HOPE (NCT 03036813), a randomized, double-blind, placebo-controlled, multicenter trial. Patients were randomized to voxelotor 1500 mg (N=90), 900 mg (N=92), or placebo (N=92). The median age was 24 years (range 12, 64). The primary efficacy outcome measure was Hb response rate defined as an Hb increase of >1 g/dL from baseline to week 24. The response rate for voxelotor was 51.1% (46/90) compared to 6.5% (6/92) in the placebo group (p<0.0001).”
Fast forward to the middle of December when the following headlines hit:
-The Committee for Medicinal Products for Human Use of the European Medicines Agency adopted a positive opinion recommending marketing authorization for Oxbryta tablets for the treatment of hemolytic anemia due to sickle cell disease in adults and pediatric patients 12 years of age and older (Stifel is modeling the first set of EU sales in Q3).
-The FDA announced it has granted accelerated approval for Oxbryta tablets to treat sickle cell disease in pediatric patients aged four up to 11 years.
While the headlines for Obryxta have been positive, the drug has not seen its full potential yet. This was made abundantly clear when BofA hosted a call with Global Blood CEO Ted Love and other members of the management team. The bottom line was that Obryxta saw a major COVID drag but the 2H is setting up to be much more favorable for the company. Mr. Love would say:
“We’ve launched really in November-December of 2019. We had almost one full quarter of launch activity before the pandemic was declared in mid-March of 2020. The performance of the launch actually was exceeding our expectation prior to the declaration of the pandemic. Since then, we’ve almost had the rate of growth cut by 40% or more. Currently we are – have penetrated about 35% of our [prescriber] targets. Meaning that they’ve written one or more prescription and we’ve penetrated about a third of the patient pool. So, there’s still a long runway ahead. It’s just taking more time because the interactions both with physicians and patients being able to visit physicians is much slower than it would be without the COVID.”
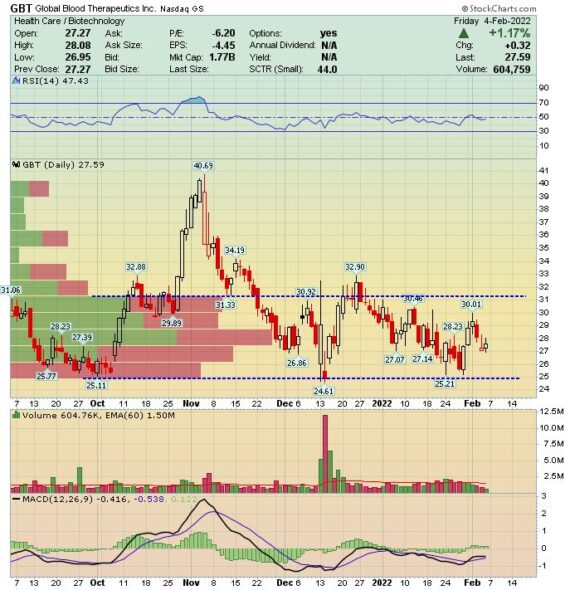
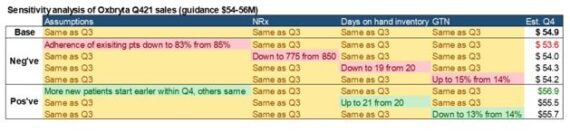
While most commentary suggests a 2H ramp, we still need to focus on the upcoming Q4 earnings report (date not confirmed). Jefferies was out on January 28th where they introduced their Oxbryta tracker. Analyst Akash Tewari said he built a bottom-up tracker for Oxbryta sales which matches well to the reported sales. According to the model, they believe Oxbryta Q4 sales will be $54.9M in a base case where the assumptions, NRx, channel inventory and GTN stay flat as Q3. Additionally, they did a sensitivity analysis by adjusting individual variables:
ASH Data
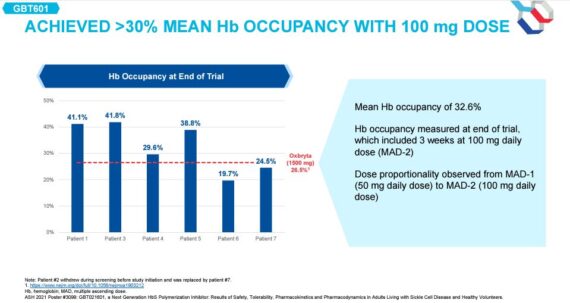
Finally, it should be noted that in December at the American Society of Hematology (ASH), Global Blood presented initial 601 data, a next gen polymerization inhibitor (PI) that looks to improve upon the profile of first-gen Oxbryta. While data from this presentation was small (6 adult patients), it demonstrated efficacy across key data points including: 1) hemoglobin response – 100% of SCD patients achieved > 1g/dl increase in Hb vs. Oxbryta where adults in registration trial 50% met this threshold and 2) Hb occupancy – a surrogate for % of pts with normal functioning Hb, where it saw 33% 601 Hb occupancy compared to 27% for Oxbryta. JPMorgan analyst Tessa Romero would say, “Ultimately, we look to further clarity on next steps on the path forward for the asset and visibility on what the next milestone(s) could be to better assess value potential within the landscape and what the next value creation event might look like.”