MedTech Monitor – IRadimed (IRMD)
IRadimed (IRMD) develops, commercializes and markets MRI-compatible medical devices that can be used safely on patients undergoing MRI procedures. It currently markets MRI-compatible IV infusion pumps and patient vital signs monitors. The company makes sales direct in the U.S. and through distributors internationally.

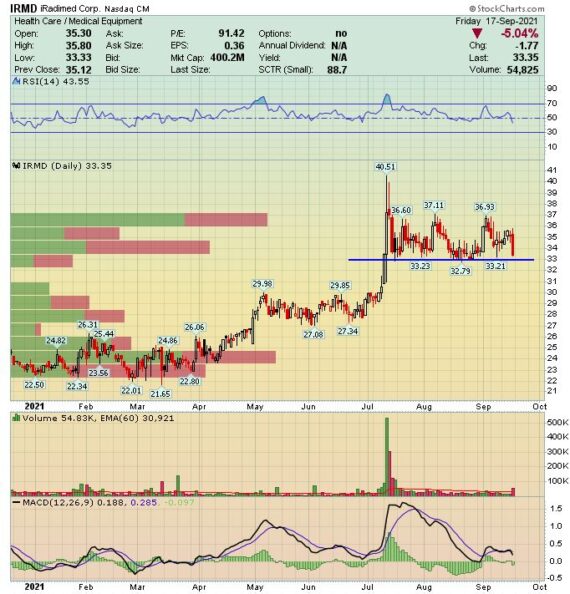
Back on July 30th, the company reported solid Q2 numbers:
-EPS of $0.14 vs $0.09 estimate – Beat
-Revenue of $9.8M vs $8.46M estimate – Beat
-Revenues increased 44% Y/Y and 6.4% Q/Q
-Domestic Revenue (82% of Total Revenue) increased 73.2%
-International Revenue (18% of Total Revenue) decreased 17.9%
-Device Revenue increased 53.4%
-Disposables and Service Revenue increased 37.7%
-Sees Q3 EPS of $0.16 – $0.17 vs $0.13 estimate – Beat
-Sees Q3 Revenue of $10.3M – $10.5M vs $9.37M estimate – Beat
-Sees FY EPS of $0.60 – $0.62 vs $0.53 estimate – Beat
-Sees FY Revenue of $40M – $40.4M vs $37.52M estimate – Beat
The average selling price of the company’s MRI-compatible IV infusion pump system during Q2 was approximately $41,600 compared to approximately $30,200 for the second quarter of last year. This increase in ASP relates to higher domestic unit sales and a favorable product sales mix as more customers purchased optional features during the current quarter. Meanwhile, the average selling price of their patient vital signs monitoring system was approximately $39,700, compared to approximately $30,600 for the same period in 2020.
CEO Roger Susi would add that another positive trend is that bookings for the quarter remained impressive and set a new high watermark for the company. “This not only allowed us to exceed our internal forecast, but also give us visibility and confidence for the back half of the year and going into the anticipated launch of our next-generation IV pump. Further, we expect bookings to remain strong for the second half of the year as we continue to gain more access to our customers.”
CFO Chris Scott did comment that the company was seeing higher materials costs, some stretching of lead-times and difficulty in sourcing certain parts. Unfortunately, this trend remains and they continue to put an increasing amount of energy in managing through these global shortages. However, despite these challenges, they said they have not yet experienced a significant impact on their ability to source materials. “We continue to see cost increases typically ranging from approximately 10% to as high as 30%, as well as greater enforcement of tariffs by our vendors. To-date, we have been able to digest these cost increases without a significant impact to margin.”
During the Q&A, management acknowledged that they will see some normalization of pump sales in the second half. “We were monitor-heavy this quarter, second quarter and in the first half. But when we take a look at our order book and the plan going forward, we do see some normalization of the IV pump business.”
Regarding disposables and services, management said this line is going to continue to be strong. “We’re seeing higher IV set sales, but also remember now wrapped up in that line item, are the disposables related to our monitor. And as that business continues to grow, we’re seeing more and more disposables — monitor disposables roll out the door as well. So, we expect continued strength on that line item.”
Product Pipeline
The company now has a ferromagnetic detection device that it released last quarter to its area leaders and now to its entire sales group. “Since then, interest in the product has been positive and earlier this month we received our first order for three units. We are encouraged by the quick interest shown in this device and expect a growing number of bookings with more time for our sales team to develop additional opportunities.”
Lastly, they have their next-generation IV pump which they are now in frequent communication with the FDA on. Based on their recent interactions, they continue to believe that clearance could come as early as December this year or early next year.
This past week, Roth Capital analyst Scott Henry was out with a note after meeting with IRMD management this next-gen pump was discussed. Takeaways included:
-The FDA appears particularly tough on approval of 510(k)’s for new pumps. Note that the last pump IRMD received approval for took 250+ FDA review days. Based on this feedback, Roth expects that the approval of the next-generation MRI IV infusion pump will likely occur in 1H22 (versus their prior target of late 2021/ early 2022).
-There are approximately 4K IRMD pumps in the market place that are 5+ years old (typical life of an IV pump is ~5 years). Replacing approximately 1K of these pumps every year could double the current pump sales run rate. Roth believes that a new pump model could expedite this transition.
-Finally, Roth’s understanding continues to be that the new pump will be easier to use for those that are familiar with other pump manufacturers. The interface should be more self-explanatory and require less ramp-up time on the new pump (their impression). This could result in faster adoption by new customers.