Pulmonx (LUNG) – Commercial Traction
Earlier this month, on November 4th, a Home Page article (See HERE) was published that provided an overview of this new publicly traded medtech company, who provides a minimally-invasive treatment for severe emphysema patients. Since that time, the company has reported quarterly earnings while also presenting at a Healthcare Conference so I wanted to pass along an update.
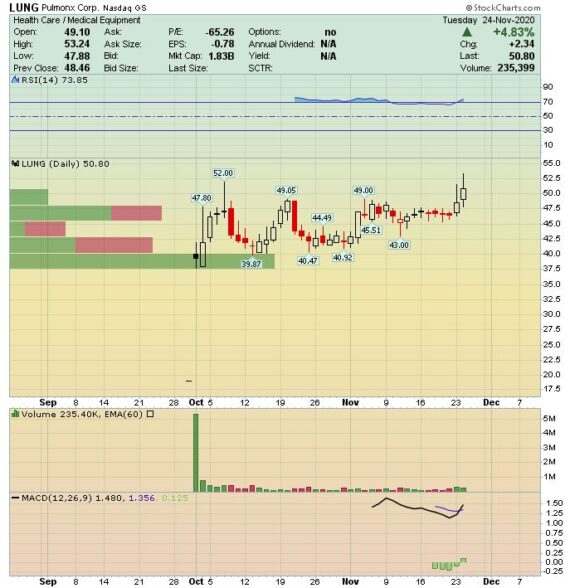
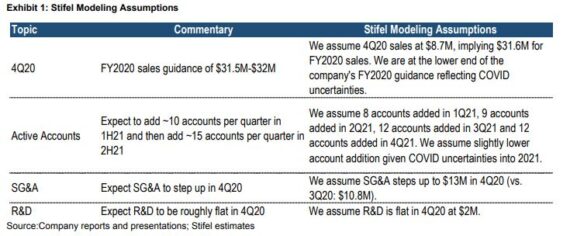
Kicking this off with Q3 earnings on November 10th, the company reported that total worldwide revenue came in at $10.6M, an increase of 17% Y/Y. Breaking that down by geography, U.S. Revenue came in at $5.3M, a 57% increase Y/Y while International Revenue was $5.3M, an 8% decrease Y/Y. The increase in U.S. revenue was driven by increasing commercial traction from the launch of Zephyr Valve while the decrease in international revenue was due to the impact of the COVID pandemic. In its press release, while the COVID-19 pandemic poses a continued risk of uncertainty to operating results, the company expects full year 2020 revenue to be in the range of $31.5M – $32M.
Stifel analyst Rick Wise, who has a Buy Rating and $50 price target on the stock, was out with a post-earnings note adding the following:
-The company added 21 active accounts in the quarter.
-While Europe sales could remain challenged due to rolling COVID resurgences/ lockdowns, management highlighted early encouraging trends that could suggest cases have peaked in some geographies (France and Belgium).
-Beyond the Zephyr valve procedure for qualifying collateral ventilation negative patients, the company is exploring expanding into treating the collateral ventilation positive patient population with AeriSeal. On the post earnings conference call, management provided key updates on clinical activities for AeriSeal. The company has started to initiate sites for the multi-national European clinical trial, but anticipates potential COVID-related delays in enrollment. In the US, the company is in discussions with FDA and anticipates IDE approval in 2021. Following the significant clinical activities ahead, Pulmonx targets AeriSeal to be available in the US in late 2024 and in Europe “sometime before then.”
-They believe numbers could prove conservative, but potential upside depends on the uncertain path of the COVID-19 pandemic. “Our 4Q20 sales estimate at $8.7M is at the low end of management’s FY2020 guidance range ($31.5M-$32M) reflecting our near-term conservatism as COVID resurges worldwide.”
Now, turning to November 16th where CEO Glen French and CFO Derrick Sung spoke at the Stifel Healthcare Conference, here were the items discussed:
-Despite their still-cautious COVID outlook, Pulmonx management highlighted a few ways the company is actively managing through the pandemic. Pulmonx has been able to significantly increase their account base and geographic diversity, which allows the company to better manage rolling COVID hotspots. Since the onset of the pandemic, the company has added greater than 20 accounts vs. 15-20 new accounts per quarter pre-COVID.
-In the near term, management highlighted their focus on establishing centers of excellence, building patient referral networks and utilizing direct to patient education. On building patient referral networks, management noted they have been able to reach more physicians through virtual interactions vs. pre-COVID.
-Management highlighted Europe and Asia as important geographies for future expansion. While Pulmonx products have been launched in some European countries for a period of time already, the company plans to do a re-launch with their newly compiled data and guidelines inclusion in these select countries. As well, the company continues to launch in new European geographies, noting a recent launch in Belgium. As Asia is in the early stages of expansion, the company noted they are seeing good adoption in China and are looking to receive approval/reimbursement in Japan in 2023.