Renalytix AI (RNLX) – Gathering the Intel
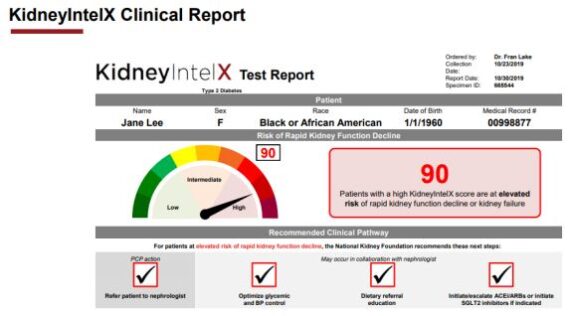
For those that do not know, Renalytix AI (RNLX) is an artificial intelligence-enabled in vitro diagnostics company, focused on optimizing clinical management of kidney disease. Its diagnostic platform, KidneyIntelX, uses a proprietary artificial intelligence-enabled algorithm that combines diverse data inputs, including validated bloodbased biomarkers, inherited genetics and personalized patient data from Electronic Health Record (EHR) systems, to generate a unique patient risk score (Shown Below). This patient risk score predicts progressive kidney function decline in chronic kidney disease (CKD), allowing physicians and healthcare systems to optimize the allocation of treatments and clinical resources to patients at highest risk.
This stock was covered in the August 23rd W/E Research and recently, we have received several questions on this name. Therefore, I wanted to provide an update especially after the company reported its fiscal Q4 numbers last week. The most recent quarter has been especially busy for the company as it announced a number of operational highlights (Shown Below):

In their post-earnings note, Stifel highlighted that following the commercial launch a month ago, the company received the first set of KidneyIntelX orders during the quarter – from 30 physicians and 20 specialists within the Mount Sinai health system. Management noted that the test is “up and running” in multiple Mount Sinai-affiliated sites, and that implementation is generally on track – though navigating early logistics challenges often associated with test launches (i.e. tech integration) has required some elevated effort. The company continues to anticipate the ordering group of physicians at Mount Sinai, as well as other healthcare providers (i.e., RNLX signaled three significant health system implementations in the intermediate term), to grow and drive revenue generation in FY21.
Catalysts
Reimbursement – As highlighted in the company’s report, RenalytixAI is actively engaged in efforts to achieve commercial coverage and reimbursement for KidneyIntelX. In February 2020, it received certification to ISO 13485 for the Salt Lake City Laboratory. In FY20, KidneyIntelX was granted a common procedural terminology (CPT) code and received its first positive coverage determination from a private insurance payor group. In addition, the Centers for Medicare and Medicaid Services (CMS) set the national price for KidneyIntelX at $950, effective on 1 January 2020. This price will remain in effect for a three-year term from January 2020 until December 2022. Stifel analyst Daniel Arias said they he continues to be focused on new business development that can expand the addressable patient population for the KidneyIntelX assay. Management remains confident in having at least two additional partners (likely a payor or additional hospital system) that can be announced over the course of the next 12 months.
AstraZeneca – The company recently announced a collaboration with AstraZeneca to develop strategies to improve patient outcomes for cardiovascular, renal and metabolic diseases, with an update on the program expected in early 2021.
COVID-19 – In conjunction with COVID-19 efforts, RenalytixAI continues to investigate the use of KidneyIntelX for patients with COVID-19 through MASKeD-COVID, a multi-center study ran alongside Mount Sinai and several academic institutions, with the goal of improving the understanding of mechanisms of COVID-19-associated kidney disease. “Initial research findings are expected in late 2020, with the commercial launch of KidneyIntelX in the COVID-19 population in 2021.” In addition, JPMorgan analyst Tycho Peterson notes that Kantaro, the JV between RNLX and Mount Sinai, continues to make progress with COVID-SeroKlir and COVID-SeroIndex, with both antibody tests recently granted CE mark approval, while Bio-Techne is set to assist with the manufacturing and global distribution of the antibody kits, with a target of 10M tests per month.