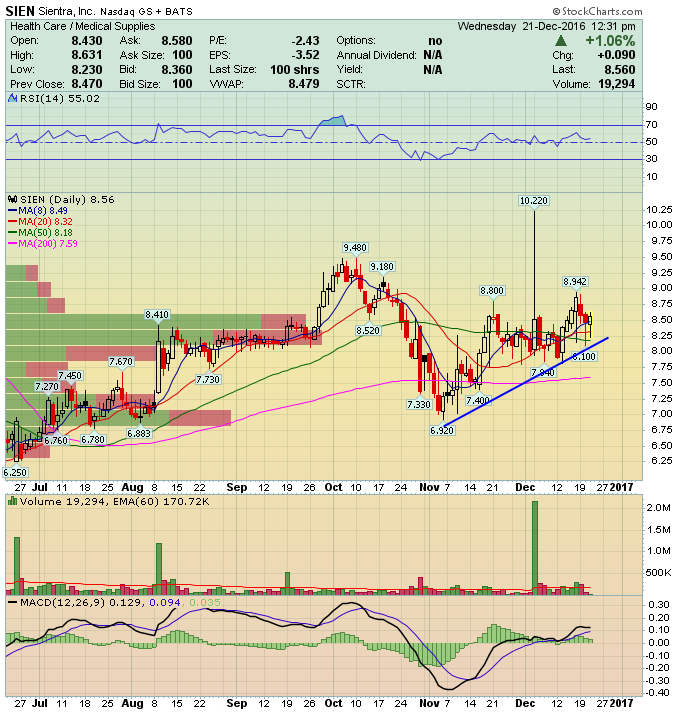
Sientra (SIEN) – Real and Spectacular?
In 2003, Sientra, a medical device company that develops and markets products focusing on the plastic surgery and aesthetics market, was founded. Sientra would become the exclusive distributor of Silimed implants in the United States. It received its initial FDA approval for its round and shaped implants in 2012 and started selling implants to plastic surgeons. Here is the company’s implant portfolio:
The company’s entry into the market broke the existing duopoly of implant manufacturers in the U.S. Prior to Sientra, all implants used in the U.S. were sold by Mentor, a subsidiary of Johnson & Johnson (JNJ), and Allergan (AGN), the makers of BOTOX. In October 2014, the company would finally go public.
Unfortunately, the company would soon be in deep water. In late-September 2015, shares of Sientra plummeted after its sole manufacturer, Silimed, had its marketing certificate suspended by U.K. and EU regulators after inspectors found that the surfaces of some devices were contaminated with particles at its Brazil facility. Brazilian regulators then shut down Silimed’s factory in the country for precautionary reasons. The following month, Sientra announced that it was temporarily putting a hold on sales of its breast implants while it sorted out all of its problems.
In November 2015, the company announced Jeffrey Nugent would become its new Chairman and CEO. A medical device and pharmaceutical industry veteran, Mr. Nugent was the founder and CEO of Precision Dermatology (later acquired by Valeant Pharmaceuticals (VRX)), and was the interim-CEO for BIOLASE (BIOL). In a statement, Mr. Nugent said, “Our decision to place the voluntary hold on Sientra products was difficult, but we felt it was the responsible action to take at the time amid the speculation, to ensure that Sientra products remain a safe choice for our customers and their patients.”
No reports ever came to light that these particles that were found caused any harm to patients. It turned out to be a non-issue from a safety standpoint. However, months after the scandal, Sientra would release its quarterly earnings that showed how much of an impact this scandal had hurt them. It announced that revenues fell to just $1.5M, compared to $12.1M a year prior. Net losses jumped from $3.2M during Q4 of 2014 to $28.3M.
Fortunately, in February of this year, the company announced that it would start selling its breast implants again on March 1st, 2016. Then, this past August, the company announced an agreement with a domestic manufacturer to make its implants. They will use Vesta, a unit of Ohio-based tech conglomerate, Lubrizol LifeSciences, which is owned by Berkshire Hathaway. CEO Jeffrey Nugent said, “Our plan is to work with them closely to establish the manufacturing operations required to meet our (Food and Drug Administration approval) specifications.” The companies hope to submit paperwork to the FDA for approval in early 2017. The FDA has a 180-day review process and Sientra hopes to have implants made by Vesta on the market late next year.
These catalysts were also pointed out in the December 19th Stifel note after their MedTech Madness Bus Tour:
“In the coming quarters, important milestones for Sientra include submitting the PMA Supplement for its new manufacturing plant with Vesta, obtaining PMA-S approval before YE17 and resolving any outstanding legal issues. Management remains highly confident it will obtain PMA-S approval before YE17 and referenced a solid working relationship with the FDA. International remains a long-term opportunity for Sientra (and not currently in our model), which could start to materialize as early as 2018.”
Also, in the chatroom yesterday, Fahad highlighted all of the option activity that has recently taken place:
• Seller of 6,000 July 7.5 Puts for 0.45 -0.95 on 12/16
• Seller of 2,000 July 7.5 Puts for 0.70 on 12/19
• Seller of 1,000 July 7.5 Puts for 0.70 on 12/20