DexCom (DXCM) Bulls Betting on Positive AdCom and Label Expansion
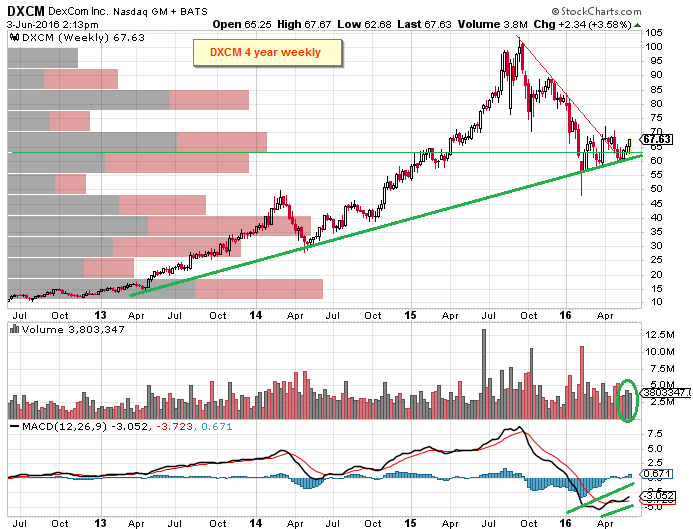
Recent options activity
On June 2nd
- 2,000 September 55 puts sold to open
- 2,000 December 70 calls bought to open
On May 26th
- 1,500 July 70 calls bought to open
Average daily options volume is 492 total.
Possible Catalysts for Options Activity
– Upcoming DexCom presentations
Any positive data at either of these conferences could send the shares higher.
- June 7, 8:00am (EDT) Jefferies Healthcare Conference in New York City
- June 15, 5:40pm (EDT) William Blair Annual Growth Stock Conference in Chicago
– FDA Medical Devices Advisory Committee Meeting
This would be the main catalyst. An approval by the committee could have a massive positive impact on the share price.
At this time, all CGM devices require a confirmation from a finger-stick test for blood-sugar level readings, and DexCom has been trying to gain FDA approval to label the meters for dosing.
-On July 21, 2016, the committee will discuss, make recommendations, and vote on information regarding a premarket approval application panel-track supplement for a proposed change in intended use of Dexcom G5® Mobile Continuous Glucose Monitoring System (CGM) device so that, in addition to tracking and trending interstitial fluid glucose concentrations, patients can use the device as a replacement for their blood glucose meters and make treatment decisions based on the interstitial fluid glucose concentration reported by the CGM.
The company believes there is a good chance the FDA will grant them the right to remove that testing requirement and that, in turn, would clear the way for Medicare reimbursement. This would be a huge win for DexCom, bringing in a large client base that is unable to afford the devices without coverage.
To learn more about our approach and how you can become a successful trader, sign up for 2 week trial and test drive live chat room with some of the best traders: SUBSCRIBE
A little background
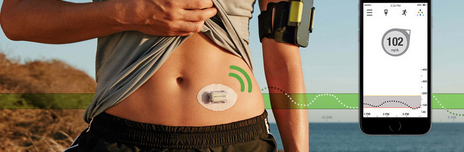
DexCom (DXCM) is a medical device company that focuses on the design, development and commercialization of continuous glucose monitoring systems (CGM) in the United States and internationally. Intended for Type 1 diabetics, their products enable continuous monitoring of blood sugar levels via a tiny flexible probe inserted under the skin, in contact with interstitial fluid concentrations. The sensor sends the data via secured wireless Bluetooth connection from the receiver to a mobile application. The patient also has the option of sharing the data with up to five people, for example parents of diabetic children.
The monitoring system presents users clear, chartable and usable data, as well as variable warning level settings and audible alerts. The DexCom G5 model has been approved by the FDA for use in both adults and children from age of 2. It is also approved for use in Europe.
On April 28th, DexCom reported a Q1 loss of 23 cents/share, a penny more than forecast, attributed mainly to higher SG&A. However, their sales increased 60% to $116.2 million. For FY2015 DexCom showed a 55% increase in sales to $402 million.
In a recent interview, CEO Kevin Sayer forecast 35%-40% growth for 2016, with revenues near $550 million.
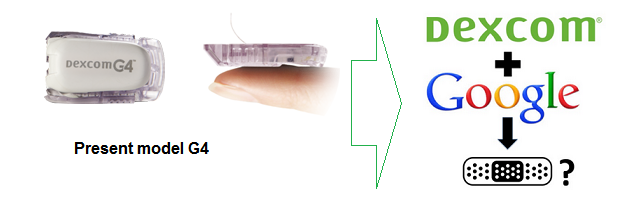
Google Collaboration
DexCom and Verily Life Sciences (formerly Google Life Sciences) entered into a collaboration agreement in August 2015 to jointly develop a series of next-generation CMG products. The main intent is, with the help of Google’s extensive technological expertise, to miniaturize the monitor/sensors into the size of a band-aid and extend the life of the sensor itself from today’s 7 days to 10 or 14. Anticipated development timeline is two to three years.
DexCom has also launched products with Johnson & Johnson (JNJ) that have been very well accepted in Europe (Animas Vibe), Tandem Diabetes (TNDM) and Bigfoot Biomedical. These partnerships are mainly associated with automated insulin delivery systems (insulin pumps).
There are approximately 300,000 Type 1 diabetes patients using CMG devices in the USA, 115,000 with DexCom and the balance with Medtronic (MDT). With Medicaid acceptance, better private payer coverage and practitioner awareness, CEO Sayer says “we’d be very disappointed if we didn’t get to 70% to 80% penetration in the type 1 market for CGM over time”. For DexCom, that would represent over 420,000 patients, calculated from today’s numbers and market share.
Dexcom ultimately hopes their next-generation products could make CGM more user-friendly and less obtrusive, helping to extend the technology beyond type 1 patients and into type 2 diabetes with over 29 million patients in the USA and nearly 410 million patients worldwide, hospitals, gestational diabetes, and perhaps even prediabetes.
Their total addressable market is potentially huge.