IPO Watch – SI-BONE (SIBN)

Set to debut this week with its lead underwriters Morgan Stanley and Bank of America Merrill Lynch, SI-BONE is a medical device company that uses a proprietary minimally invasive surgical implant system called iFuse, to fuse the sacroiliac joint to treat sacroiliac joint dysfunction that often causes severe lower back pain.
SI-BONE was founded in 2008 by orthopedist Mark A. Reiley, M.D., the main inventor of iFuse, as well as President, CEO, and Chairman, Jeffrey W. Dunn, and orthopedic surgeon Leonard Rudolf, M.D. Dr. Reiley previously invented balloon kyphoplasty and founded Kyphon Inc., which was sold to Medtronic in 2007. He also invented the INBONE total ankle replacement system, which was sold to Wright Medical Technology in 2008.
The iFuse Solution
According to SI-BONE, the iFuse procedure is typically performed under general anesthesia. The surgeon uses a custom instrument set we provide to prepare a triangular channel for each implant through the ilium, across the sacroiliac joint, and into the sacrum. An iFuse implant is then pressed into the triangular channel, which is slightly smaller than the implant, creating what is known as an interference fit. The triangular cross section of our iFuse implants, as shown below, prevents them from rotating. Our triangular iFuse implants cross the sacroiliac joint and provide immediate joint stability, which is why we believe pain diminishes soon after the iFuse procedure. Over time, bone grows onto the implants and across the joint, permanently stabilizing or fusing the joint.
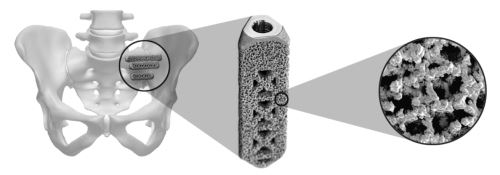
The company also has its second-generation iFuse implant, called the iFuse 3D, shown on the left below, that was cleared for marketing by the FDA in March 2017 and the European Union in May 2017. This patented titanium implant combines the triangular cross-section of the iFuse implant with a proprietary 3D-printed porous surface and fenestrated design. This design also allows the surgeon to fill the implant with ground-up bone before implanting it, which some surgeons believe accelerates bone through-growth. iFuse-3D implants have shown positive bony ingrowth in cell culture and animal studies, whether or not ground-up bone is used, as shown in two peer reviewed studies published in June 2017 in the International Journal of Spine Surgery. The image on the right below shows the cross section cut from an iFuse-3D implant removed from an animal as part of the study, and reveals robust growth of bone into the implants.

Market Opportunity
According to the S-1 filing, the company estimates that over 30 million American adults have chronic lower back pain. For patients whose chronic lower back pain stems from the sacroiliac joint, their experience in both clinical trials and commercial settings indicates that iFuse could be beneficial for at least 30% of patients who are properly diagnosed and screened for surgery by trained healthcare providers. Approximately 282,000 patients in the United States were estimated to have received multiple non-surgical steroid injections for sacroiliac joint pain in 2017. Based on market experience and internal estimates, and the assumption that the average person suffering from sacroiliac joint dysfunction has been in pain for five years, SI-BONE estimates that the potential market for iFuse in the United States could be 279,000 patients annually, for a potential annual market in the United States of approximately $2.7 billion.
Since January 1st, 2018, because of the strength of published clinical evidence on iFuse, 18 U.S. payors have published reimbursement policies exclusively covering the patented triangular design of the iFuse implants and excluding coverage of other products that are intended to fuse the sacroiliac joint. SI-BONE believes that the full impact of each exclusive coverage decision grows over time as they continue to educate surgeons about the coverage and the medical criteria they need to follow, and train them on the diagnosis and how to perform the iFuse procedure.
In 2016 and 2017, SI-BONE generated revenue of $42.1M and $48.0M, respectively, a growth rate of 14%. Gross margins were 88% and 89% for 2016 and 2017, respectively. Finally, the number of iFuse procedures performed in the six months ended June 30th, 2017 and 2018 was 2,739 and 3,200, respectively.
