Unlock: Heron Therapeutics (HRTX) Trade Idea
(This HRTX Trade Idea was issued on July 30 for $3.50. The October 35/50 Call Spread was closed today for $7.00 or 100% gain)
Heron Therapeutics
Ticker: HRTX
Sector: Biotech
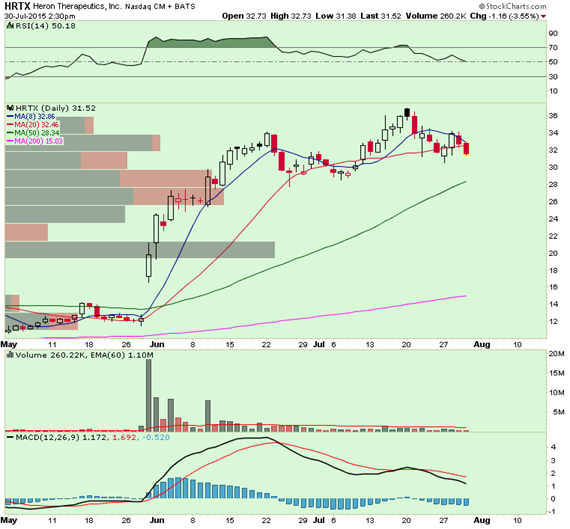
Current Price: $31.50
Target: $50.00
Stop Loss: $25.00
Time Duration: 78 Days
Trade Idea: Buy HRTX October 35/50 Call Spread for $3.50 or less.
Large bulls in the option market are gradually rolling positions out from September to October 35 calls for the last few weeks as open interest in October 35 calls has climbed to 9,000+. Today, one trader closed 2,000 September 25 calls for $7.75 and bought 3,300 October 35 calls for $5.25. About $1.5 million call premium rolled out. Expect the open interest to climb even higher tomorrow.
HRTX is $1.1 billion development phase biotech with a product called Sustol for the prevention of acute chemotherapy nausea and vomiting. Extension of this drug is to treat post-surgery acute pain conditions. Baker Brothers own 2.9 million shares, which increased last quarter from 2.6 million shares. Other major shareholders include Point72 with 500,000 shares. Back on June 2, large insider buying noted when Director Kevin Tang bought 150,000 shares for $19.30 to $23.84. On March 19 the company reported solid clinical data for Phase 1 study of HTX-011 for prevention of post operative pain. Then on March 28 they reported Phase 3 MAGIC Sustol study met primary end point.
The progress in clinical studies has been incredible. But we believe the stock has a lot more to go. Upcoming catalysts:
Next Few Weeks – FDA acceptance of Sustol NDA submission. Note if approved, this will differentiate Sustol as the only 5-HT3 receptor antagonist approved for this indication, which we view as critical to HRTX’s ability to command a premium price for Sustol
By August 31 – HTX-011 Phase II Dose-Ranging Data
By September 30 – HTX-011 Phase II Proof of Concept Data
The key here in short term is Sustol. Longer term HTX-011 comes into play as well. We believe Sustol will get approved. The clinical trial was 90% powered for a 10% relative difference in response rate and was able to show a 14.3% relative difference. The secondary endpoint of patients achieving “complete control” was also statistically met and the percentage of patients who experienced no nausea or infrequent nausea during the delayed-onset phase met as well. Also, statistically, more patients in the Sustol arm were satisfied with their therapy, based on a QOL questionnaire. Importantly, there were no clinically significant differences in the rate or severity of adverse events between the treatment arms. No acute HEC results were shared, but management claimed that there was a highly consistent benefit that either statistically met or strongly trended in favor of Sustol.
The study was powerful and outcome was excellent. The FDA should accept the NDA with final approval in Q1 of 2016.