Behind The Numbers – STAAR Surgical (STAA)
STAAR Surgical designs, develops, manufactures and markets implantable lenses for the eye and companion delivery systems. The company provides Visian implantable collamer lenses (ICLs) to treat visual disorders, such as myopia, hyperopia, astigmatism, and presbyopia; and Hyperopic ICL, which treats far-sightedness. It also offers intraocular lenses (IOLs), including collamer material and silicone foldable IOLs, and nanoFLEX IOL that produces a clearer image, as well as pre-loaded injectors for use in cataract surgery.
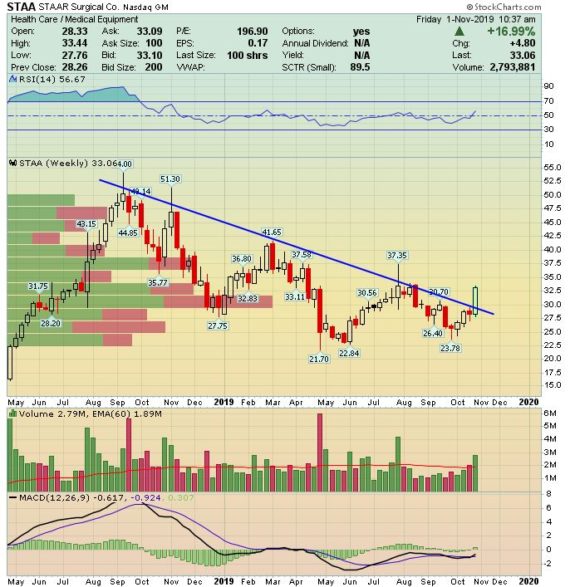
This is a name Jaguar clients are familiar with as we have discussed it a handful of times, with the most recent being back in April. On Wednesday, after the close, the company reported their Q3 earnings which then saw its stock rise by 17% the next day.
-EPS of $0.05 vs $0.05 estimate – In-Line
-Revenue of $39.1M vs $38.56M estimate – Beat
-Net Sales increased 23% Y/Y
-28% ICL Revenue Growth
-35% ICL Unit Growth
CEO Caren Mason added that on a regional basis, ICL unit growth in the third quarter successfully hurdled record year-ago levels in several markets, with Japan units up 57%, China units up 48%, South Korea units up 38%, France units up 20%, Spain units up 14%, UK units up 13% and Germany units up 11%. Also, commercial activities during the quarter designed to support the final week of the high implant season in China included mobile and social media marketing for consumers.
In addition, they initiated new marketing programs in Europe that will soon include a very exciting and new influencer campaign in Spain. They also began advertising in the U.S. in September with billboard and YouTube advertising to extend and amplify ICL digital banner and clinic marketing in the Midwest.
Regulatory Matters
Turning to the company’s regulatory activities, in Europe, their EVO EDOF lens for presbyopia remains under review by DEKRA, their notified body. They continue to anticipate this product will be on the market in the second quarter of 2020 in CE Mark countries, opening a new and large market opportunity for STAAR that expands the target age range for their EVO family of lenses.
Meanwhile, in the U.S., the company received a letter from FDA dated October 25, 2019, approving their supplement seeking approval for the clinical trial for their EVO family of lenses in the U.S. “The letter included a few additional study design recommendations, which we are working to include in the study protocol. We are responding to FDA within a week or so. We continue to qualify study sites and expect our time lines will not be impacted as we work to close out the study design, including FDA’s latest recommendations. We very much look forward to moving expeditiously through next steps and enrolling patients.”
Upcoming Events
Next week, on Friday, November 8, it will be hosting its invitation-only 2019 Institutional Investor and Analyst Day in New York City, where they look forward to outlining their strategic vision for the 2020 to 2022, 3-year planning period.
Caren Mason, on the conference call, added, “At our Investor Day, we are going to have a number of proof points either through video and/or through surgeons who are in the room, who are present, who will be talking about why the future of refractive surgery is lens based. That is really the theme of the next 3 years in terms of the strategic direction for this business. We have a number of strategic partners around the world who are already there, and we have many lined up who are excited for the future, having the majority of their business move into lens based. We will have examples from surgeons in the United States who are moving into 70% plus ICL practices with no laser vision correction equipment present in their new business model.”
Finally, on November 13, they will be meeting with investors at the Stephens Annual Investment Conference in Nashville, Tennessee. They will also be meeting investors at their Lake Forest corporate headquarters during the fourth quarter.