NeuroPace (NPCE) – Seizing an Opportunity
NeuroPace (NPCE) is a $236M medical device company focused on people suffering from epilepsy by reducing or eliminating the occurrence of debilitating seizures. Epilepsy is a devastating chronic disorder characterized by a tendency of the brain to produce sudden abnormal bursts of electrical energy that disrupt brain functions and cause seizures. Epilepsy is classified into two main categories– focal epilepsy and generalized epilepsy. Approximately 60% of epilepsy patients have focal epilepsy, which is characterized by electrical discharges that originate in a specific part of the brain. The remaining 40% of patients have generalized epilepsy, which is characterized by widespread electrical discharges that involve the entire brain at once.
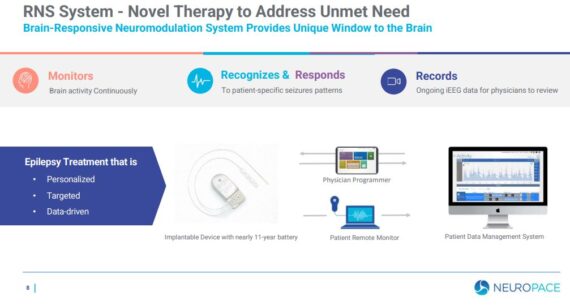
The company offers the RNS® System, the first and only commercially available, brain-responsive platform that delivers personalized, real-time treatment at the seizure source. “By continuously monitoring the brain’s electrical activity, recognizing patient-specific abnormal electrical patterns, and responding in real time with imperceptible electrical pulses to prevent seizures, our RNS System is programmed by clinicians to deliver the precise amount of therapy when and where it is needed and provides exceptional clinical outcomes with approximately three minutes of stimulation on average per day. Our RNS System is also the only commercially available device that records continuous brain activity data and allows clinicians to monitor patients not only in person, but also remotely, providing them the data they need to make more informed treatment decisions, thus optimizing patient care.”
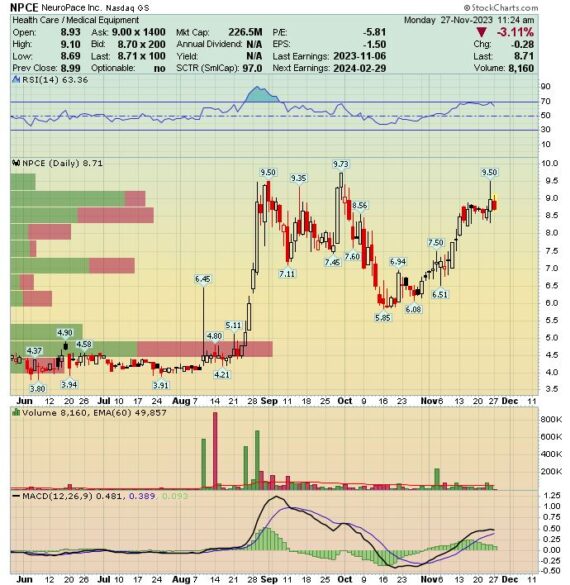
Earlier this month, the company reported its Q3 earnings in which total revenue came in at $16.4M, up 47% Y/Y while gross margins came in at 74.5% vs 71.4% last year and 72.5% last quarter. Meanwhile, the company would increase their FY guidance to $62.5M – $63.5M vs previous guidance of $59M – $61M. On the call, management would add they recently introduced 2 new product enhancements designed to streamline the RNS experience. They launched their enhanced nSight data management system with overwhelmingly positive clinician feedback. They also recently launched their new Tablet Remote Monitor, or TRM, ahead of schedule. “The TRM and nSight launches enable important advancements in the efficiency and ease of use of the RNS system.”
In their post-earnings note, Morgan Stanley would highlight that the quarter’s performance was driven by new patient implant and the DIXI distribution agreement. FY23 revenue guidance was raised by $3M, implying Q4 revenue of $15.6M (+21% Y/Y). “This appears conservative given the past two quarter’s performance excluding replacement revenue, and improvements in the diagnostic funnel that should further support initial implant growth.”
Catalysts
New CEO – Back in June, the company hired Joel Becker as its new CEO. Mr. Becker recently worked as the President of Viking North Ventures, which specializes in working with medical technology and healthcare companies, Boards and Investors to accelerate growth by providing advisory, leadership and strategy development services. Prior to Viking North, Mr. Becker served as President of the Cardiac Rhythm Management and Neuromodulation business for Integer Holdings. Prior to Integer, he spent more than 15 years at St. Jude Medical where he served as President of the Americas Division and led the commercial organizations for the electrophysiology, cardiology, structural heart, cardiac rhythm management and neuromodulation businesses in the Americas.
Most recently, Wolfe Research held their Annual Healthcare Conference in which Mr. Becker gave his “fresh takes” on the business? According to analyst Mike Polark, “Significance of opportunity, capability of the technology, and knowledge of the RNS team is impressive. Epileptologists approach RNS on a continuum: some consider RNS application only in single therapy setting, others apply it with a hybrid strategy (drugs + RNS, surgery + RNS), and still others do not understand the modern RNS story. In the last group, company needs to continue to highlight the positive evolution of the RNS platform and ensure practitioners understand the recent improvements including battery longevity, long term outcomes data, and the digital monitoring and engagement capabilities.”
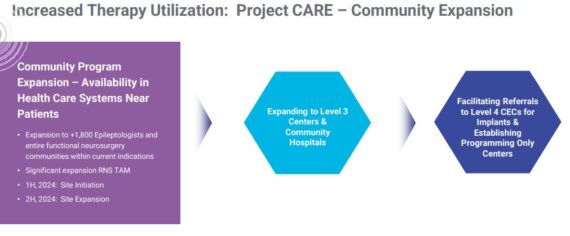
Project CARE – This catalyst is also referred to as their community opportunity. This community epilepsy center initiative will allow NeuroPace to expand beyond level 4 centers, effectively widening the referral pathway beyond and speeding up the time from diagnosis to RNS treatment. On their Q3 conference call, management would say:
“We have been focused on refining our strategy for launching our commercial efforts into the community and are expecting full launch of our pilot program with a group of community customers in the first half of 2024. We have seen significant interest in these efforts, and some of these pilot customers have been eager to advance quicker through the process. As a result, we’re happy to announce our first implants with the RNS system in the community setting with this initial group of pilot customers. The patients implanted are doing well, and we are pleased that we are now able to bring RNS therapy not only to the additional 1,800 epileptologists in an expanded group of functional neurosurgeons, but most importantly, to the indicated patients who would or could not have been referred to a Level 4 center for treatment.”
According to Wolfe Research, management’s estimate of the opportunity is large. Of the 1.2M drug resistant epilepsy patients, management estimates around 700K suffer from focal epilepsy but only 50,000 of these patients are treated in level 4 centers each year. As NeuroPace increases engagement with community sites, this is likely to aid prospects of helping RNS address the other 650k.
Recent Partnership – Over a year ago, NeuroPace entered into a commercialization agreement with DIXI Medical. Under the terms of the agreement, NeuroPace will have exclusive rights to promote and sell stereoelectroencephalography (SEEG) electrodes in the U.S. DIXI will continue to support U.S. physicians as well as education and outreach activities to inform patients and HCPs of the treatment options available for drug refractory epilepsy. SEEG electrodes are used in the epilepsy monitoring units of comprehensive epilepsy centers to determine where epileptic seizures originate. Physicians use this information to target interventional treatments at the seizure source, including with the NeuroPace RNS System. At the Wolfe Healthcare Conference, management said they were pleased with the commercial, strategic, and revenue contributions to date. The partnership gives NeuroPace a structural reason to engage further upstream (phase 2 monitoring) in the diagnostic process.

NAUTILUS – Finally, a much longer-term catalyst surrounds the company’s NAUTILUS research study, which is evaluating the safety and effectiveness of thalamic stimulation with the NeuroPace RNS System as an additional treatment in reducing the frequency of primary generalized seizures in people 12 years and older with drug-resistant idiopathic generalized epilepsy. Specifically, this study will involve participants with IGE whose seizures have not been controlled by antiseizure medications and who currently have generalized tonic clonic seizures, with or without myoclonic or absence seizures. On the Q3 call, management said they were pleased with their continued progress in enrolling patients into the trial and they remain on track to complete enrollment in Q1 2024.